Abstract
Background: Hematopoietic cell transplantation (HCT) is currently the only cure for sickle cell disease (SCD), with excellent survival using a matched related donor (MRD). Disease specific HCT registries have significantly advanced understanding of outcomes, via more comprehensive data collection in a larger number of patients. Such data may provide long-term outcomes after HCT, resulting in identification of advantages and disadvantages over conservative therapy, helping to focus future efforts on avoiding or improving the latter.
Objective: To investigate the long-term outcomes of HCT in pediatric SCD, related to transplant, disease complications, and organ function.
Methods: A multicenter retrospective registry of transplanted SCD patients was created through the Sickle Transplant Alliance for Research (STAR). Baseline, transplant, and long-term (most recent if >2 yrs from HCT, otherwise 1 yr) outcome data was collected from 10 centers. Disease recurrence was defined as acute SCD symptoms with hemoglobin S >50%. Graft rejection was defined as whole blood or myeloid donor chimerism <5%. Pulmonary function testing values were converted to functional categories per previous reports. Pre- and post-HCT values were compared using McNemar's test and extensions. P-values <0.05 were considered statistically significant.
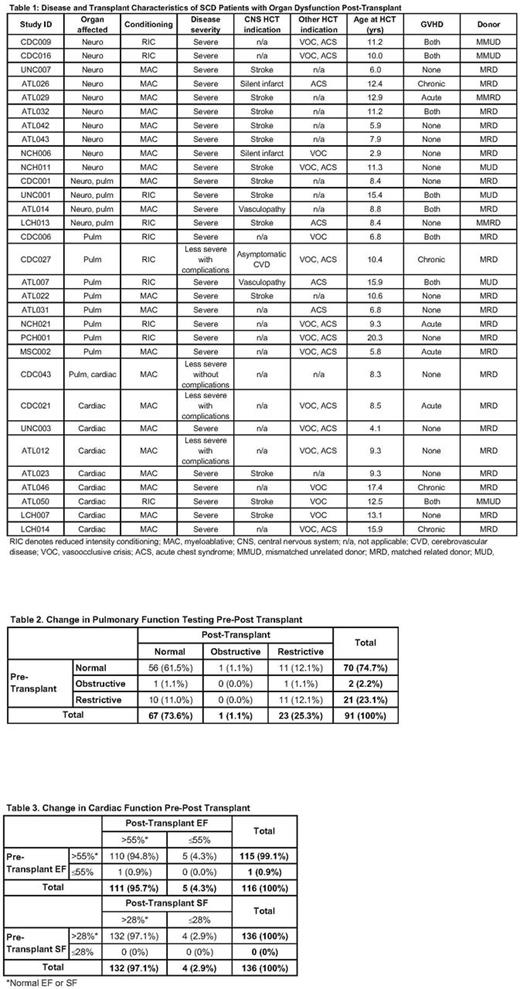
Results: Overall, 174 of 196 patients survived ≥1-yr post-HCT, with last assessment at 1186 d (Interquartile range, IQR: 760, 2351). Of these, 70.4% underwent HCT for clinically severe SCD from 1993-2016 (median, 2011), at 9.8±5.2 yrs (range, 1.6-25.6). Seventy-five percent (n=131) of patients received stem cells (87.0% marrow) from a MRD, and 58.1% received myeloablative conditioning, 93.7% with in vivo T cell depletion. Fourteen patients (8.1%) had rejection/disease recurrence 0.8-3.4 mo post-HCT; of these, 7 underwent a 2nd HCT. Overall, 45 patients (25.9%) developed chronic GVHD, at a median of 226 d (IQR: 140, 336), which was severe in 11 (24.4%) and extensive in 5 (11.1%). Ten patients required systemic immunosuppression 15-39 mo post-HCT, while 157 patients discontinued such at a median of 237 d (IQR: 173, 408). Donor chimerism was maintained long-term at a median of 100% in unsorted (n=101) and sorted (T: n=85, myeloid: n=69) samples, as reflected in median post-HCT hemoglobin levels of 13.0 g/dL (n=169; IQR: 11.8, 13.9). Posterior reversible encephalopathy syndrome (PRES) was reported in 18 patients (10.3%); 12 developed seizures with PRES and 9 without (12.1% with seizures). Of the 105 patients with available pre- and post-HCT brain MRIs, 2 developed new infarcts, 6 had new vasculopathy, and 10 had progression of previous infarct or vasculopathy; all but 2 of these patients had CNS disease pre-HCT (Table 1). While there was no difference between pre- and post-HCT pulmonary functions (P=0.79; n=91), 10 patients normalized and 11 had deterioration (Table 2). In the 13 patients with either a restrictive or obstructive pattern post-HCT, 47% had acute chest syndrome as an indication for HCT. One patient with poor cardiac function transitioned to normal function post-HCT, while 5 had a lower EF (p=0.103) and 4 a lower SF (p=0.05) post-HCT (Table 3). All patients who developed an abnormal EF or SF received myeloablative conditioning (88.9%) or underwent HCT for clinically severe SCD (66.7%). Mean TR jet velocity was normal in the 64 patients that underwent this evaluation post-HCT. Immune reconstitution was robust, with median ALC 2587 (n=151; IQR: 2000, 3357) and present/normal splenic uptake on nuclear medicine scan in 90.9% (n=44). Bone age was delayed in 44.9% (n=69). Bone mineral density showed a mean spine and body Z score of -0.56 and -0.50, respectively (n=92). In post-pubertal females, 22 had normal menstruation (48.9%) and 23 menstrual irregularity (51.1%), with 13 on hormone replacement therapy; data was not obtained in 15.
Discussion: In this comprehensive retrospective registry with detailed long-term data, we were able to identify transplant-related sequelae, albeit in a minority of patients. Progression of SCD and HCT-related complications, though rare, merit careful follow up for potential intervention. Our data supports the establishment of prospective long-term registries post-HCT to ensure adequate healthcare, more accurate identification of the risks and benefits of transplant for SCD, and opportunity to improve outcomes.
Kanter: Apopharma: Research Funding; Sancillo: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; American Society of Hematology (Sickle Cell Disease Guideline Panel): Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; MUSC: Other: The site PI for sponsored research conducted at MUSC who receives funds from: Novartis, bluebird bio, GBT, Sancillo, Apopharma, Pfizer; GBT: Research Funding; NHLBI (sickle cell disease research advisory committee): Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding. Guilcher: Jazz pharmaceuticals: Consultancy; Canada Market Research: Consultancy; Patient Access Solutions: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal